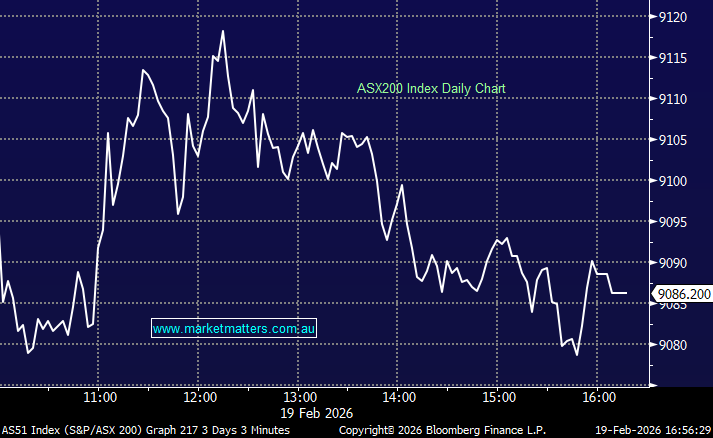
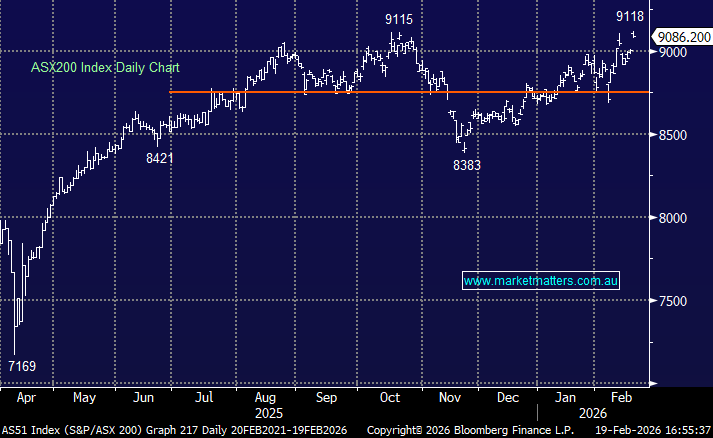
Updated view on Telix Pharma Ltd (TLX)
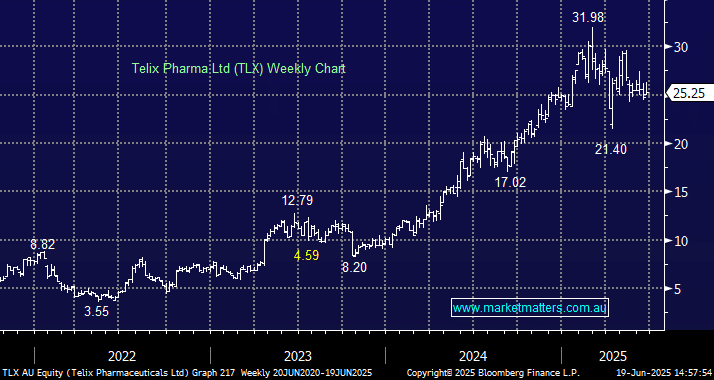
Good afternoon MM team, I would like to know your most recent views on Telix Pharma. The company seems to be having some early success with the commercialisation of a few products; however most recently, the FDA did not approve their next product in the pipeline (Pixclara), and commercialisation has been delayed. I also heard (appreciate if you could confirm) that there might be unfavourable US taxation regime changes on the way for companies such as Telix and CSL, and that is also putting pressure on the share price for both companies. Looking at the financials for Telix, I was a bit surprised to find that their operating margins are just in the low teens. For comparison, Neuren is about 80%, while CSL is close to 33%. Return on capital and ROE are also much lower for TLX. Probably not a fair comparison, due to a different business model, which would be great if you could help me understand. Thanks, Angela